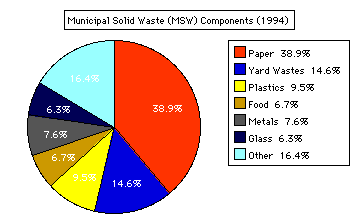
1994 Municipal Solid Wastes (MSW) Composition
proposed by, Bruce Hoglund, February
1998
Background
“More than 209 million tons of MSW was generated in 1994. Paper
and paperboard accounted for 81.3 million tons (38.9 percent) of the
total waste stream, yard wastes 30.6 million tons (14.6 percent),
plastics 19.8 million tons (9.5 percent), metals 15.8 million tons
(7.6 percent), food 14.1 million tons (6.7 percent), glass 13.3
million tons (6.3 percent), and other 34.2 million tons (16.4
percent) (Figure 8).”1
“The generation of MSW has increased from 88 million tons in
1960 to 209.1 million tons in 1994. During that time, per capita
generation of MSW increased from 2.7 pounds per person per day to 4.4
pounds per person per day (Figure 9). Per capita generation is
expected to remain constant through 2000, when total MSW generation
is expected to reach 223 million tons.” 2

Pollution and wastes are really nothing more than misplaced
chemicals. Recycling of these chemicals is usually limited by
economics, convention, and regulations. These constantly shifting
factors may now be favorable towards the nearly complete recycle of
waste materials. Most recycling efforts center around fairly
homogeneous materials such as steel, aluminum, paper, and glass. The
more mixed these materials are with others, the less valuable they
become. Methods such as magnetism for steel and density differences
for glass and aluminum can be employed to further separate loosely
mixed materials, although it is still best to separate these
materials as early in the waste generation cycle as possible, e.g.,
curbside recycling programs. Except for a few specific examples
(e.g., milk jugs, soda bottles, etc.) plastics do not lend themselves
to recycling, even if a ‘pure’ stream of waste plastics is
obtained. This is because plastics are not pure materials, but
complex mixes of hydrocarbons (carbon and hydrogen) and various other
radicals such as chlorine (e.g., PVCs), cyanide (CN, e.g., acrylics),
and fluorine (e.g., Teflon). It is these radicals, when thermally
broken down at high temperatures and then essentially quenched by the
quick cooling that occurs during the combustion or incineration
process that is responsible for the production and emissions of
cyanide and the greatly feared dioxins and furans. By decreasing the
peak temperatures, increasing the residence time at high
temperatures, and providing a safe molecular ‘sink’ for the
radicals (e.g., sodium) the production and emission of these
materials can be eliminated. This is why Molten Salt Oxidation (MSO)
continues to attract interest for the difficult to incinerate
materials. Thus, MSO is not a competitor of incineration, as it is so
often presented, but a complementary tool in the manipulation of
chemicals and disposal of wastes.
As can be seen in the MSW composition graph above, much of MSW is
“biomasses”. In fact, MSW is a already collected biomass
that is largely going to waste. Furthermore, it is ideally colocated
with the customers needing the energy, unlike most other biomass
harvesting schemes.
Historical MSO and Today’s World
Commercial Molten Salt utilization began with the operation of the
Hall-Héroult method of producing Aluminum over 100 years ago.
Molten salts for oxidation were first considered at the turn of the
century for the gasification of coal. The products are
“syngas” which is a mixture of hydrogen (H2) and
carbon monoxide (CO) gases. Syngas can be used directly as a fuel
gas, as it has a heating value about 1/3 that of natural gas and
cleanly burns to produce only carbon dioxide (CO2) and
water (H2O). Syngas can also be the starting point for
many chemicals, plastics, or liquid fuels (e.g., methanol). The
gasification of coal application of MSO has been rekindled
periodically, but most recently with the energy crisis of the late
1970’s and 1980’s. The main obstacle is the difficult
economics of using a low value fuel (coal) to produce a slightly
higher value product (syngas). These difficult economics are
compounded by the high as of coal and the additional cost of removing
the ash from the MSO. The ash issue is the greatest unknown in the
MSO field. Ironically however, most of the MSW ash is less tightly
bound and thus more easily pre-separated (e.g., removing cans and
bottles) than coal.
Difficult economics, not technical difficulties, stopped the MSO
gasification of coal. The ability to obtain the energy and materials
in MSW for a revenue (tipping fees) instead of a cost will provide an
obvious advantage over coal in economics of operation.
Current interest in curbing carbon dioxide (CO2)
greenhouse gas emissions via capture and sequestering and
establishment of a hydrogen economy may also be aided by developing
MSO as it can produce concentrated CO2 and H2
gases.
|
|
Percent by |
Moisture |
Dry Weight lb (based on 100 lb |
Percent Dry Weight |
Typical Density |
|
|
Density, Typical |
kg/m^3 |
|||||
|
Component |
Weight |
percent |
or kg sample) |
(%) |
lb/ft^3 |
lb/ft^3 * 16.019 = kg/m^3 |
|
Food wastes |
0.15 |
0.7 |
4.5 |
5.8 |
18 |
288 |
|
Paper |
0.4 |
0.06 |
37.6 |
48.1 |
5.1 |
82 |
|
Cardboard |
0.04 |
0.05 |
3.8 |
4.9 |
3.1 |
50 |
|
Plastics |
0.03 |
0.02 |
2.9 |
3.8 |
4 |
64 |
|
Textiles |
0.02 |
0.1 |
1.8 |
2.3 |
4 |
64 |
|
Rubber |
0.01 |
0.02 |
0.5 |
0.6 |
8 |
128 |
|
Leather |
0.01 |
0.1 |
0.5 |
0.6 |
10 |
160 |
|
Garden trimmings |
0.12 |
0.6 |
4.8 |
6.1 |
6.5 |
104 |
|
Wood |
0.02 |
0.2 |
1.6 |
2 |
15 |
240 |
|
Glass |
0.08 |
0.02 |
7.8 |
10 |
12.1 |
194 |
|
Tin cans |
0.06 |
0.03 |
5.8 |
7.4 |
5.5 |
88 |
|
Nonferrous metals |
0.01 |
0.02 |
1 |
1.3 |
10 |
160 |
|
Ferrous metals |
0.02 |
0.03 |
1.9 |
2.5 |
20 |
320 |
|
Dirt, ashes, bricks, etc. |
0.04 |
0.08 |
3.7 |
4.7 |
30 |
481 |
Percent by weight (dry basis) Component Carbon Hydrogen Oxygen Nitrogen Sulfur Ash Food wastes 48.0 6.4 37.6 2.6 0.4 5 Paper 43.5 6.0 44.0 0.3 0.2 6 Cardboard 44.0 5.9 44.6 0.3 0.2 5 Plastics 60.0 7.2 22.8 -- -- 10 Textiles 55.0 6.6 31.2 4.6 0.2 2.5 Rubber 78.0 10.0 -- 2 -- 10 Leather 60.0 8.0 11.6 10 0.4 10 Garden trimmings 47.8 6.0 38.0 3.4 0.3 4.5 Wood 49.5 6.0 42.7 0.2 0.1 1.5 Dirt, ashes, bricks, etc. 26.3 3.0 2.0 0.5 0.2 68
Chemical Composition of Individual Solid Wastes4
Current Opportunities for MSO
The most studied opportunity for MSO is the destruction of hazardous
wastes including radioactive and chemical weapons (since the 1950s by
Rockwell). Unfortunately, although commercial molten salt technology
is over 100 years old, its use for oxidation of wastes is still
relatively new, especially when compared with the millenia old
burning technology upon which incineration is based. It is our belief
that the immediate application of MSO to the most hazardous wastes is
the equivalent to attempting to run before you learn to walk. We wish
to develop practical, inexpensive MSO technology for the more common
wastes (e.g., “garbage” or MSW) or biomasses, and the
production of valuable gases and products before the more difficult,
niche services area of hazardous, radioactive, and chemical weapons
is attempted.
Basic MSO of MSW Concept
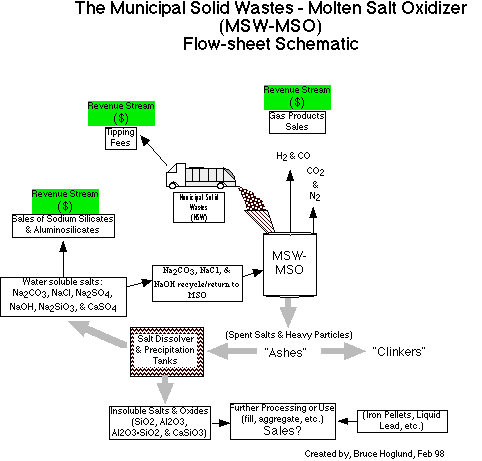
The main processes of interest occuring within the MSO are the
following:
CxHyOz + heat --> z CO + y H2 + (x-z) CC + H2O + heat --> CO + H2 (the “Water Gas reaction”)
CO + H2O --> CO2 + H2 + heat
C + CO2 + heat --> 2 CO
We can roughly say, from a rough heat balance perspective:
4 C + 6 H2O + O2 = 6 H2 + 4 CO2
‘2 1/4 parts water to 1 part carbon’, or slurry 69%
water.
The actual MSO-MSW process can be ‘black boxed’ and
considered by the following rough flows out of the MSO facility and
the following facts:
Salt facts:
Heat content about equal to water on volumetric basis.
Density of about 2.3 that of water.
Conducts electricity
Corrosive
Excellent Solvent
Viscosity at operational temperature similar to water’s
Catalytic Properties
MSO Facts:
Thermal efficiency: 70%
Energy Product Produced: Hydrogen gas
All carbon is ultimately oxidized to CO2
What uses do the 2 gas streams (CO & H2 and
N2 & CO2) have? What markets (profitable
uses)?
What is the current make up of the trash to a waste to energy
plant (e.g., the Ogden Waste to Energy Plant in Alexandria, VA)?
How much MSW is processed there? What are its energy balances?
Do they presort the MSW they receive? What do they remove? Does the
removed material have a value? If so, what are the materials and how
much is obtained? What are the costs of the pre-sorting operations
($/tonne of ‘raw wastes’).
How much ash is removed? What is the composition of the ash? Does it
have a value/use?
What are the best combination of methods of removing ash causing materials in the trash?
Aqueous? (Hydropulper, Density difference, etc.)
Air density? (Air Knife, etc.)
Mechanical (Tableing, Tromell, Screens, etc.)
What would be the losses of sodium if the salt removed is 20% ash
and the ash is in the form of 90% albite
(NaAlSi3O8) and 10% anorthite
(CaAl2Si2O8).
What is the likely ash causing content of a pre-sorted MSW feed
into the MSO?
What is the likely salt removal rate (tonne salt/tonne waste
feed)?
What is the heat losses of the salt removal?
What are the ash costs (salt losses) of:
Tires
Magazines (kaolin coatings)
“Juice Boxes”
What are the values of the product gases; hydrogen, N2
& CO2?
Who are the biggest customers of these gases?
Question:
If we have a 1,000 tonne/day MSW plant receiving ‘typical’
garbage (Alexandria example?), what are the component flows?
Example:
Gas Products production Hydrogen produced/day? CO2 & N2
produced/day?
Glass removed Glass entrained (fed into MSO)
Al cans removed Al entrained
Iron removed Iron entrained
Dirts & other unclassifieds removed Entrained
Total landfill requirements (tonnes/day)?
Salt recycle information:
Sodium is added as a NaOH & NaCl mixture, where the NaOH comes
from electrolysis of brine and the main cost is the electricity.
Sodium Chloride costs: $50/tonne
Electricity cost: $0.03/kW-h
NaOH cost: 1 tonne 70% NaOH/2500 kW-h
Question:
What by-products of the NaOH production could be sold? How much
byproduct(s) are produced? How much do the by-product(s) sell for? To
whom is likely to buy these byproduct(s) {who are the main consumers
now}?
MSO Ash Facts:
MSO Ash causing elements, in materials containing the following:
Silicon SiO2
Calcium CaCO3
Aluminum Al & Al2O3
Iron Fe & Fe2O3
Sulfur
Completely undesireable:
Mercury
Arsenic
Zinc?
Cadmium?
Ash is the key to economic MSO-MSW operation!
Main (MSO) ashes are:
What are the main ash forming materials in MSW?
If we must remove salt that has a 20% ash content;
What amount of salt is removed/day?
What is the likely (20%) ash portion’s composition?
Assuming equlibrium chemical compositions at 900° C, what are
the likely chemical components of a mix of the MSO ash feed (see
above answer) & 50% NaCl, 30% Na2CO3,
& 10% NaOH & 10% Na2S?
How do we pre-remove the ash forming materials?
Tabling (Hand sorting)
Tromel Screens
Curbside recycling
Magnetic
Density differences
Air (knife)
Water (“floatation”)
Densified water (salt &/or 'sand' “Heavy Media”)
Biogas?
Mix post digested sewage with raw MSW & digest
Post separation of digested MSW
Heavy Media (insoluble MSO salt ash)
Light fraction feeds MSO
Fluid recycled
How about thinking entirely outside the box? (Wet & fouling are
now good)
New delivery methods
Hydropulpers?
Sewers (existing, new higher capacity short runs)?
Select clients?
Small & local combined sewage & MSW MSO plants?
Do hydropulpers allow easier separation? At feed site, or pre-MSO feed?Additional products
Sewage (raw, biogassed, or secondary digested)
Wood Pulping operations?
Refinery wastes?
Fouling wastes in general
1 Energy Information Agency (EIA) branch of Department of Energy (DOE), article, “Municipal Solid Waste Profile: Introduction”, URL: http://www.eia.doe.gov/solar.renewables/renwable.energy.annual/contents.html
3
Tables’ data from "SOLID WASTES:
Engineering Principles and Management Issues", George
Tchobanoglous, Hilary Theisen, Rolf Eliassen (1977).
Pages 59 - 63, Table 4-4 "Determination of Moisture Content for Solid
Wastes Same in Example 4-1" Page 60, TABLE 4-6 Typical
Densities..."